Biological ageing - this is the way
Biological or cellular ageing is driven by a number of mechanisms. As our understanding of those mechanisms has improved so the promise of stopping or even reversing ageing comes closer to reality.
Summary: Our understanding of the mechanisms of how we age is steadily improving. We give a quick introduction to four such mechanisms: Transcription/translation errors ('copying too quickly'), telomere shortening ('when the cap no longer fits'), oxidative stress ('I'm gonna steal your electrons'), mitochondrial dysfunction ('running out of energy') and the consequence of those mechanisms - senescence ('building up trash').
Why this is important: The whole approach to health and well-being could move from one focused on treatment to prevention; from allopathic (focus on specific ailments) to holistic. If we do start living longer, and in better health, there will be knock-on effects to society, family, education and productivity.
The big theme: The ongoing survival of the human species is one of the ultimate sustainability themes. Understanding the broad spectrum of influences on human ageing can help us understand areas for investment as well as individual choices that we can make.

The details
Ageing, or rather slowing or even reversing ageing, is receiving increasing investment - $5.2 bn in 2022 - in areas including renewal therapies, diagnostics, precision medicine, drug discovery platforms and immunology. Other relevant, and maybe not so obvious areas, include diet and mental fitness.
We briefly introduced the landscape of ageing in this blog 👇🏾

We shall be looking at this area in much more depth, bringing together these insights together with other areas in a Deep Dive - 'The Death of Ageing' which will be published soon. In that blog we shall explore some potential solutions to slow down and even reverse ageing at the cellular level.
The mechanisms that cause us to age are highly complex, and involve factors both within and outside of our control.
Understanding the cellular and genetic mechanisms of ageing, however, gives us a foundation to not only fight disease, but potentially increase the length and quality of our lives.
What is happening in the depths of our cells and DNA - our genetic blueprint - the processes that are currently a natural part of the ageing process for most living organisms, may hold the answers to fighting ageing and prolonging lifespans.
So let's start by taking a look deep inside our cells, and inside the centre or nucleus of our cells to see what makes them tick.
What is going on in our cells?
Caveat: the human cell is more complex than the representation and descriptions below, but I have simplified it so that we can focus on areas and structures relevant to our discussion. It is not meant for microbiology or cellular biology experts!
Cells house the biological machinery that makes the proteins, chemicals, and signals responsible for everything that happens inside our bodies. Here is a simplified diagram:
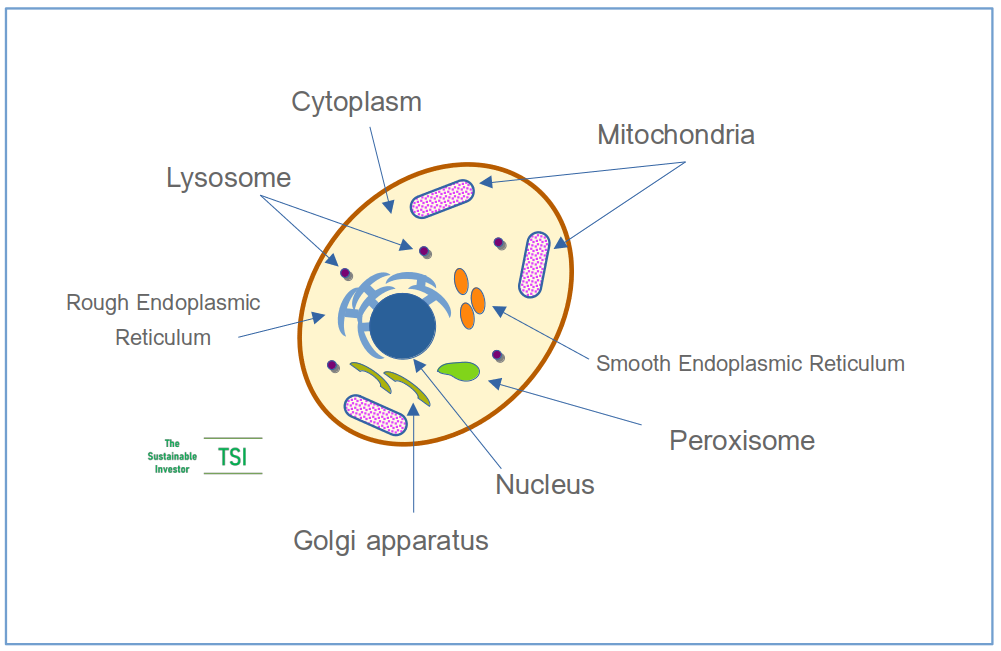
The mitochondria are the powerhouses of the cells. They finish off steps that begin in the cytoplasm of the cell to produce the energy that enables the cells to produce the proteins and chemicals for our bodies - cellular metabolism.
The other elements, with the exception of the nucleus, are involved in the manufacture of those proteins and chemicals and in getting them ready to be 'shipped' outside of the cell, as well as cleaning up, detoxifying and recycling material.
The nucleus (the blue circle in the middle of the cell diagram above) contains the blueprints / genetic instructions - the DNA - which is arranged into larger structures, called chromosomes. The common ‘X’ configuration is a result of proteins called histones holding the DNA in nucleosomes and bundled together into a form that can fit into our the nucleus.
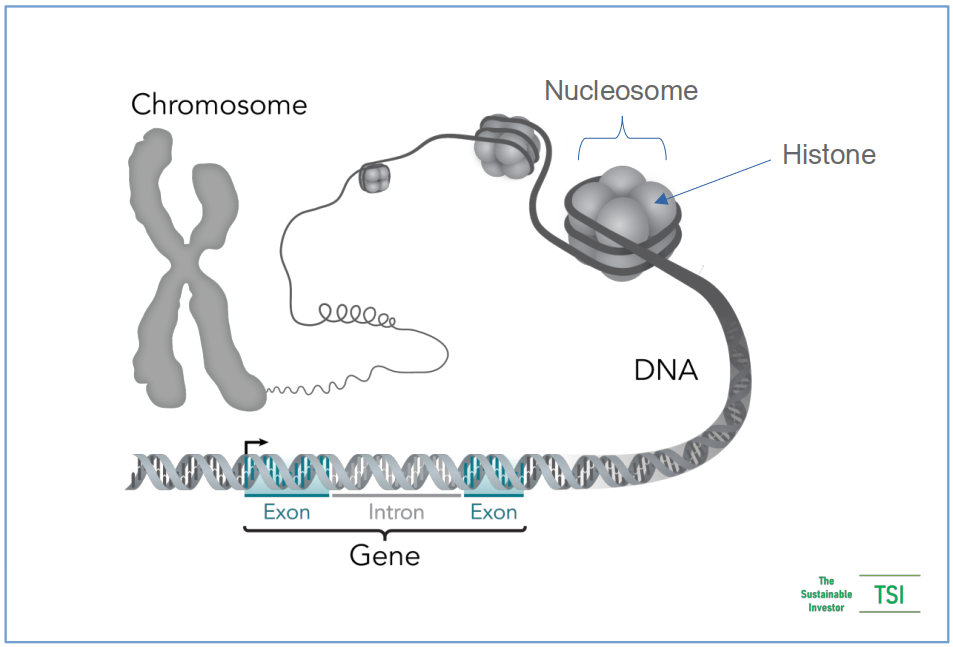
Unravelling the strands of DNA we find that they form a 'double helix' structure.
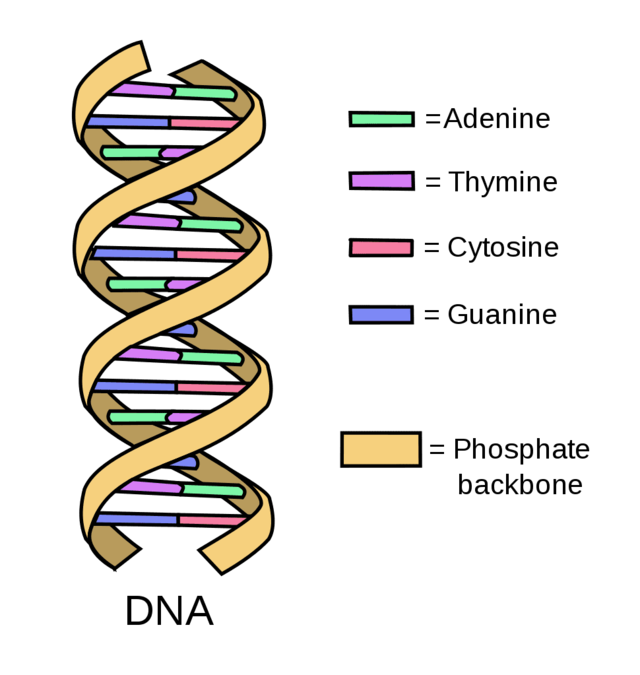
DNA is made up of four building blocks called nucleotides: Adenine (A), Thymine (T), Cytosine (C) and Guanine (G). They form pairs through chemical bonds between each spiralling helix - A with T and G with C.
Genes are made up of sequences of DNA that contain information for making specific proteins that ultimately express particular physical characteristics like brown eyes, for example.
In order to sustain life, we must continually multiply and replicate our cells, which involves replicating our DNA in addition to using our DNA to create proteins and enzymes that are responsible for gene expression and our overall biological functioning.
Scientists have discovered that as we age – that is the longer our cells and in turn DNA are replicating – not only do mutations start to occur in our DNA, but also some of these processes become less efficient and less ‘correct’, resulting in abnormalities that affect how we function. In other words we age.
Let's look at some of those. Research into ageing remedies centres around addressing, correcting and even reversing some of these mechanisms.
Copying too quickly - translation / transcription
The processes by which DNA is used as a template to create RNA and in turn proteins and enzymes (used to do everything we need to do in our body) are called transcription and translation.
Essentially, a piece of DNA that codes for a specific gene is copied into something called 'messenger RNA' in the nucleus of the cell. That then carries that information out of the nucleus into the cytoplasm where the information is translated into a protein.
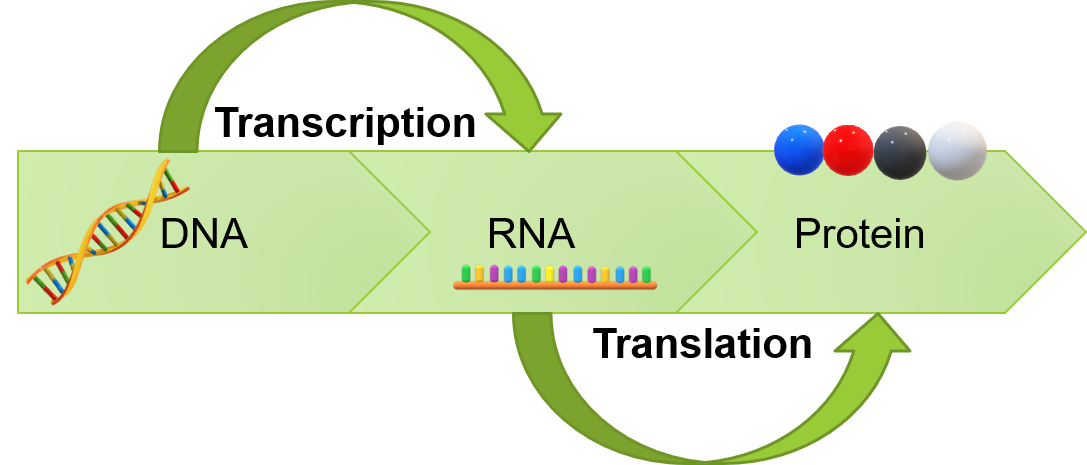
Studies have shown that the speed at which this is done increases with age, causing errors in the transcribed genetic code. If we rush things, we can make mistakes!
Some cross-species studies have shown that slowing down this process increases the accuracy of transcription and in turn translation, which was shown to increase the lifespan of the subjects.
When histones become malformed or wrongly placed, this affects the ability to replicate our DNA properly, and in turn the biological processes and viability of cells. Interestingly, studies have shown that certain dietary compounds can affect the integrity of histones.
When the cap no longer fits - telomere shortening
Looking back at the chromosome as a whole, there are sections at the end of each chromosome called telomeres, a region of repetitive DNA that is not part of our genetic code but acts as a ‘cap’ to protect the integrity of the chromosome.
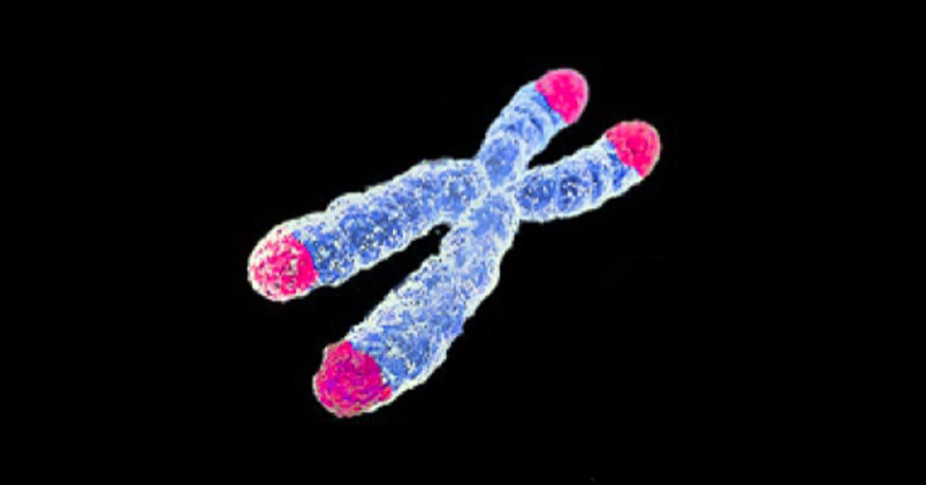
Each time a cell divides, these telomeres become shorter, eventually becoming so short that the cell can no longer divide, and ultimately dies.
I'm gonna steal your electrons - oxidative stress
A free radical is a molecule that contains oxygen but with one or more unpaired electrons - that means it is very reactive with other molecules. They can arise naturally in the body and are increased due to external factors such as pollution, sun exposure, and unhealthy lifestyles.
Note: In the diagram below the 'nucleus' is that of an atom in a molecule and not the nucleus of a cell.
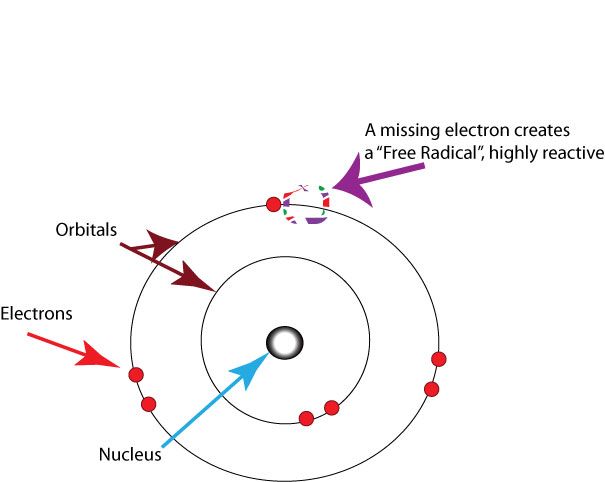
Free radicals can interact with things in the cell including DNA or proteins and 'steal' their electrons which makes the previously free radical stable but of course makes their 'victim' unstable.
This oxidative stress can lead to a chain reaction causing damage within the cell.
Running out of energy - mitochondrial dysfunction
We discussed earlier that the mitochondria produce the energy that allow the cells to function. However, there are a number of things that can hamper mitochondrial function. Oxidative stress is one (see previous section) and inherited disorders such as MELAS Syndrome.
Environmental toxins including air pollution and pesticides as well as certain viral, bacterial or parasitic infections can impair the function of our mitochondria.
Building up trash - senescence
There are many other biological processes at play that feed into the ageing process, including immunosenescence where the immune system becomes less effective through non-disease related issues. Like the ones above, they can result in dysfunctional cells and cell components that build up like trash!
These are referred to as ‘senescent cells’ and may have a key function in triggering inflammation, connected to a multitude of age-related conditions, including cancer, diabetes, osteoporosis, cardiovascular disease, stroke, Alzheimer’s disease and related dementias, and osteoarthritis. It has also been linked to declines in eyesight, mobility, and thinking ability.
These are just some of the mechanisms that current research is looking into when it comes to the science of ageing. The cells or components within the cells become damaged or hindered and are unable to produce the chemicals that they are supposed to. Ultimately, this area is a massively fertile ground for further research and broad-sector collaboration.
We shall be looking at this area in much more depth, bringing together these insights together with other areas in a Deep Dive - 'The Death of Ageing' which will be published soon. In that blog we shall explore some potential solutions to slow down and even reverse ageing at the cellular level that address the mechanisms discussed above.
Something a little more bespoke?
Get in touch if there is a particular topic you would like us to write on. Just for you.
Contact us
Please read: important legal stuff.


