
Greening ammonia - heat, pressure and hydrogen
Ammonia is an important product, particularly for the manufacture of fertilisers. It is emissions intensive, so how can we make it greener?
Summary: An important product from the chemical industry is ammonia. It's uses are varied, from explosives to smelling salts (main image) to its current primary use: nitrogen fertilisers (80%). Ammonia is treated as flammable, can be harmful to us (even fatal) and the production of it globally has a direct CO2e footprint as big as Brazil's. In terms of direct emissions, it is almost twice as emissions intensive as crude steel production and four times that of cement at approximately 2.4 t CO2 per tonne of production. What can we do to make it greener?
Why this is important: In the sustainability transition, ammonia could become even more important as a potential energy carrier too.
The big theme: The chemicals sector currently contributes more than 2 Gt CO2e per year, or close to 4% of global GHG emissions. Unlike say Oil and Gas, the demand for these products is not going to reduce materially, and we cannot just assume that better alternatives will be found quickly. As a result, transitioning the chemical sector is important, and a major challenge. There is uncertainty around the best pathway and from a financial perspective, new pathways will require very different means of production, exposing the industry to stranded assets.

The details
What is ammonia
Ammonia is a colourless gas which is about half as heavy as air and dissolves easily in water. Whilst ammonia gas itself isn't flammable, when mixed with air it could explode if ignited and so is generally treated as flammable.
Exposure to ammonia gas or solution (ammonium hydroxide) can be quite harmful to us, causing irritation at low levels but severe pain, burning, swelling and damage to skin, lungs, stomach and heart and can be fatal. The effects are usually immediate.

Why is it important? Why will it continue to be important?
About 80% of ammonia produced is used to make fertiliser which is important in helping to ensure high crop yields and aiding food security.
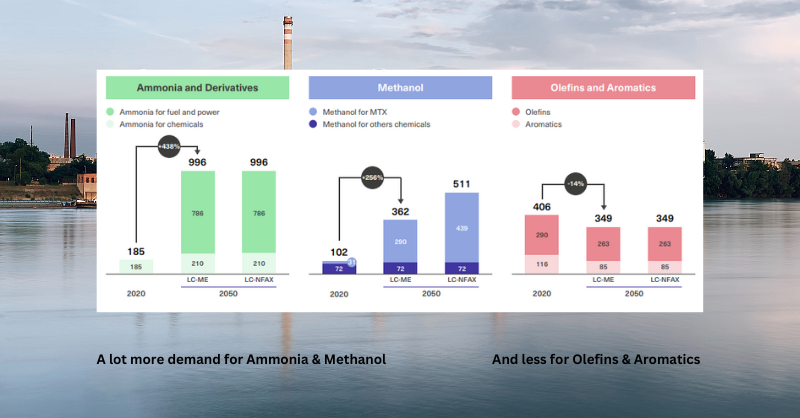
Those use cases are likely to change further over time with uses for fuel and power coming to the fore. More in this blog 👇🏾
How is ammonia produced currently?
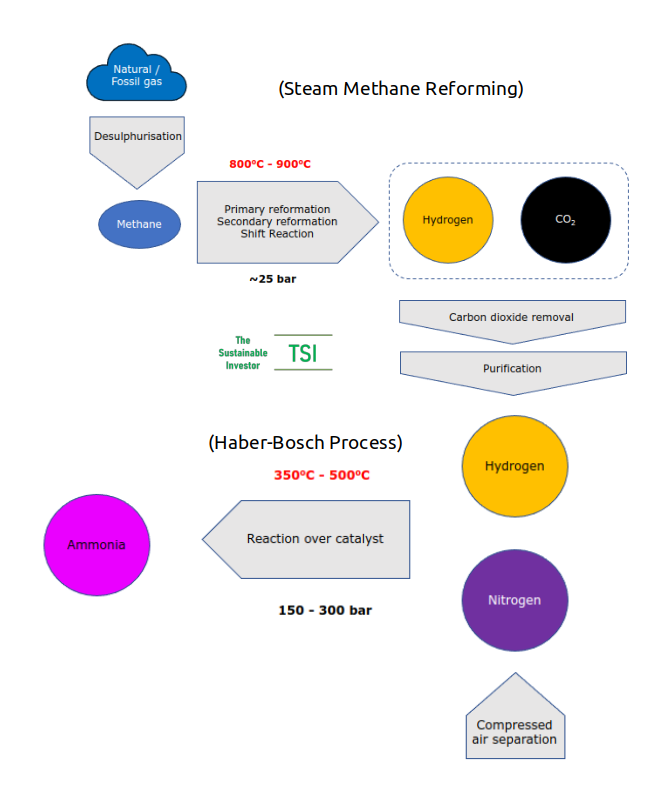
Currently ammonia is produced using the Haber-Bosch process, essentially combining nitrogen and hydrogen over an iron catalyst at a high pressure (between 150 and 300 bar or ten times the pressure in a passenger car tyre) and high temperature (between 350oC and 500oC). The chemical equation is N2 + 3H2 ➡ 2NH3 (one molecule of nitrogen plus three of hydrogen produces two ammonia).
Direct emissions from current ammonia production are 450 Mt CO2e. The majority of these come the production of hydrogen - almost no hydrogen gas exists naturally on Earth so it needs to be produced. Hydrogen is produced using a process called Steam Methane Reformation (SMR) which takes methane (from 'natural' or 'fossil' gas) and reacts it with steam under a moderate pressure (up to 25 bar) in the presence of a nickel-alumina catalyst to produce hydrogen, carbon monoxide and carbon dioxide. The reaction itself requires heat to keep it going. A lot of heat. Typically between 800oC and 900oC.
So there is CO2 from the reaction itself and also from burning fuel to create the heat needed. In addition from the extraction and transportation of the feedstock (natural/fossil gas).
About 170 Mt CO2e are indirect emissions from the generation of electricity needed to create the heat and pressure needed for the Haber-Bosch process and the chemical reaction that takes place when fertilisers are applied to soils and, largely, the nitrous oxides created.
Here is an overview:
Greening ammonia
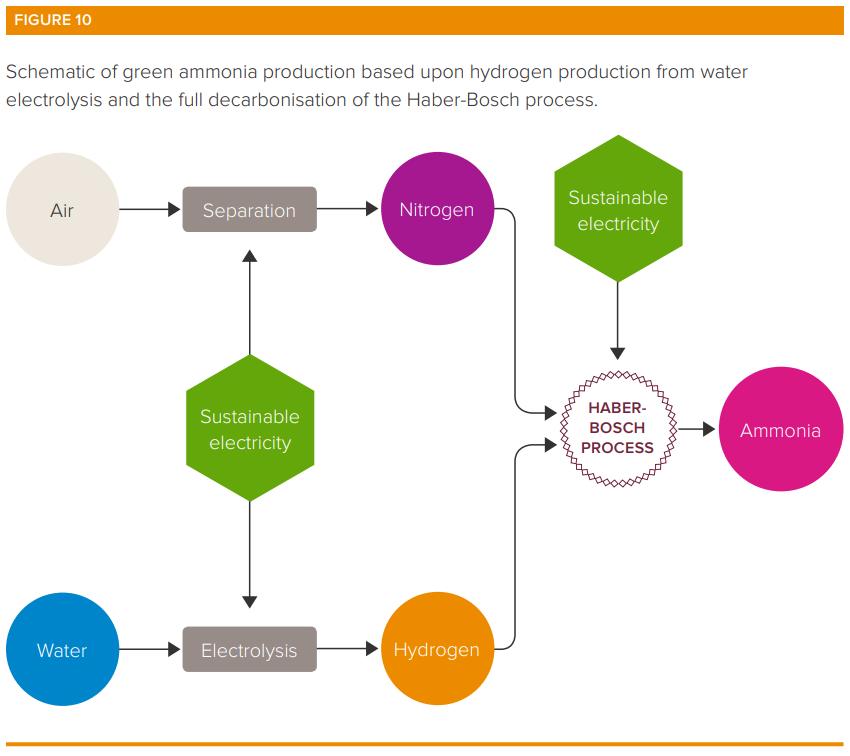
Efforts to green ammonia production chiefly centre around decarbonising the production of hydrogen. Alternatives range from SMR but capturing the CO2 produced through to using renewables to create green hydrogen through electrolysis which breaks down water molecules into oxygen and hydrogen (often brine or salt water is used producing hydrogen and chlorine - a topic for another Quick Insight).
In addition, introducing low carbon forms of heat and pressure generation from sustainable electricity.
Cost is a big consideration. Currently according to S&P Platts, hydrogen production costs range from $0.96 / kg for US Gulf SMR without any carbon capture and storage up to £13.74 / kg for UK PEM electrolysis. The cheapest green hydrogen at the moment is US Gulf alkaline electrolysis at £2.29 / kg. SMR is of course sensitive to the price of natural/fossil gas.
Alternatives to the Haber-Bosch process itself are also being looked at from exploring lower temperatures (which could bring in alternative heating sources such as industrial heat pumps) and lower pressure requirements through to utilising bacteria. In future blogs we shall be diving much deeper into the topic.
And of course, one option is to reduce the need for ammonia-based fertiliser through innovative farming practices and changes to how we eat. Only 17% of the nitrogen from fertilisers is actually consumed by humans in crops, dairy and meat products with the rest leaching out into the soil, water and air.

We shall explore green ammonia in more detail in a future long blog.
Something a little more bespoke?
Get in touch if there is a particular topic you would like us to write on. Just for you.
Contact us
Please read: important legal stuff.


